We live in an amazing world! When we dig into the details of nature, we find things that surprise us, make us curious, and often leave us bewildered. Even our best scientists only understand small pieces of the complexity of the world. I believe that there is a God who not only understands it all, but invented it all. And in my own scientific training, I have found that God thought of everything.
It is a hot Texas summer day! After working up a sweat outside, you pour a cold glass of water and drop a few ice cubes in for good measure. As you tilt your glass to take a sip, the water maneuvers around the ice pressed on your lips and down your throat. Ah, refreshing! You place your glass down and you hear the ice clinking, moving back in position atop the water. It is but a tiny detail of your life experience: ice floats.
Almost all molecules on earth are denser as solids than their liquid counterparts. In fact, that is most of the reason why solids are solid at all! But for an object to float, it has to be less dense than water. Solid ice is less dense than liquid water, allowing it to float. But why? And why does it matter?
If you only know one chemical formula, it’s water: H2O. Two hydrogen atoms and one oxygen atom. A water molecule is often described as looking like Mickey Mouse, with hydrogens like two mouse ears attached to an oxygen head.
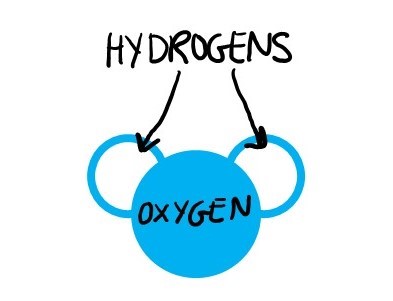
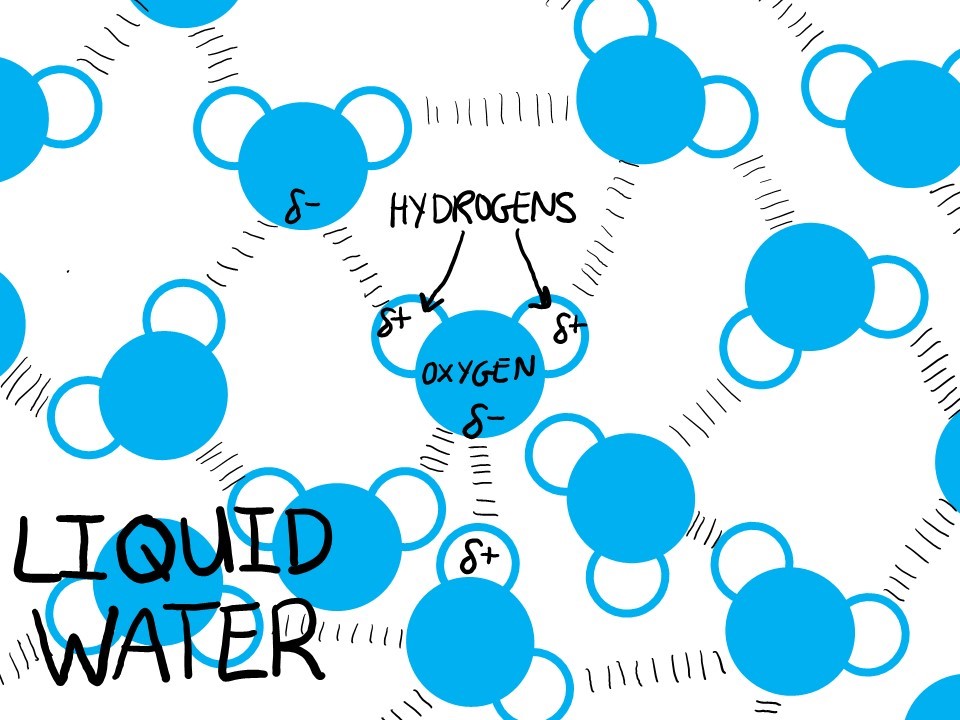
Water molecules are experts at a particular type of chemical interaction called a hydrogen bond. A hydrogen bond is an interaction between an atom that is just a little positive and an atom that is just a little negative–like a baby version of “opposites attract.” Since water has both a little positive and a little negative, its absolute favorite thing to hydrogen bond with is other waters!
In liquid form, each water molecule will hydrogen bond with 3 or 4 other waters. These bonds form and break and re-form very quickly. At a given moment, some waters have lots of space but most are very close together.
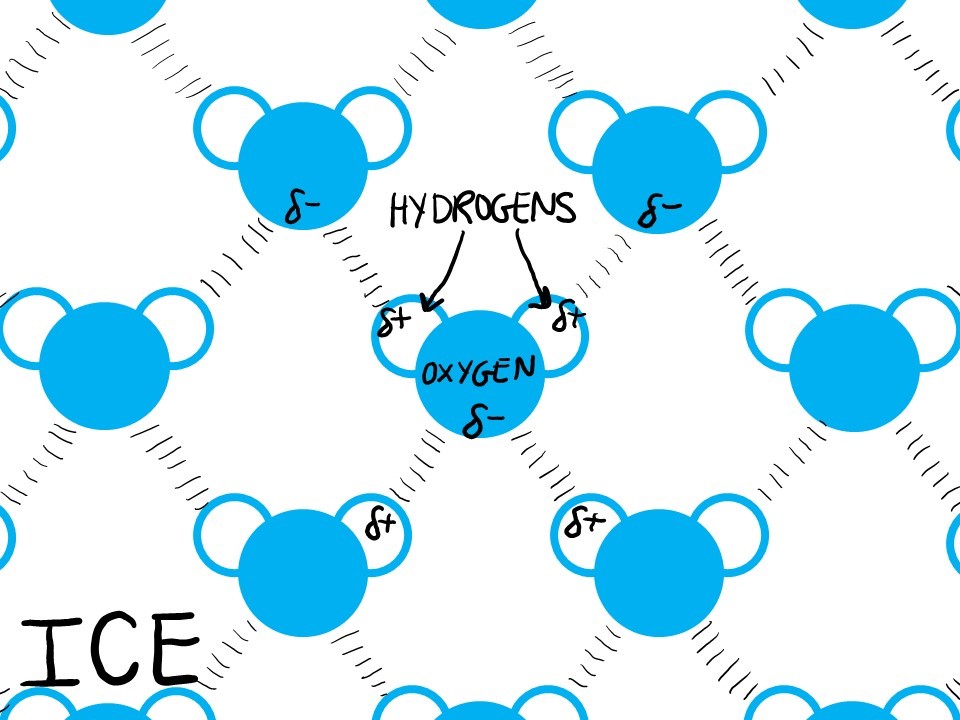
When temperatures cool, bonds do not break so easily and water molecules generally stay put. In the chemical world, more bonds means more stability. So to be stable in its solid icy form, water molecules arrange themselves such that every water molecule hydrogen bonds with 4 other waters… and this simply takes up more space than the usual dynamics of liquid water. Thus, ice is less dense than water.
It may seem like a trivial thing that ice can float, but in reality, this unique property means life or death on Earth. Because ice floats, bodies of water freeze from top to bottom. The top layer of ice actually traps heat in the water underneath and insulates it–this is why most lakes don’t just freeze solid.
If ice sank, bodies of water would freeze from bottom to top. There would be no insulating layer. Sinking ice would be replaced with new water at the top, which would freeze and sink and bring new water to the top also, until whole lakes and even parts of oceans are frozen solid. Frozen water would trap and kill sea life along with it. Could you imagine trying to recover from your entire body being frozen solid every winter?* The fishing industry in some regions would not exist even if ice-transportation was refined, since all the seafood would be frozen. Large bodies of water may never fully thaw even if they were exposed to 100 hot Texas summer days.
Even if bodies of water did not ever become giant ice cubes, sinking ice would cover the sea floor. It would crush and kill seaweeds, which significantly contribute to Earth’s oxygen supply. It would render animals such as angel sharks, flounder, and sea stars homeless since they would no longer have access to the safety of the sand. It would increase the chances of ships running aground in what appear to be deep waters. Even falling into a pool or lake could be life-threatening with potentially sharp, solid crystal formations underwater. Ice fingers in the Antarctic demonstrate the dangers of ice on the bottom of the ocean (check out ice fingers here). Ice that floats is critical to life as we know it.
Dear God, thank you for considering the need for floating ice when you wrote the rules of chemistry! Your creation includes such specific yet necessary things. Your design is perfect and it is because of you that life can flourish. Amen.
*There is a type of frog that actually does survive this by a unique mechanism–comment if you are interested in reading about this!
More about this topic:
- https://www.worldofmolecules.com/3D/hydrogen-bonds-in-water-and-ice.html (3D models
- https://courses.lumenlearning.com/boundless-biology/chapter/water/ (interactive animations)
- Why does ice float in water? – George Zaidan and Charles Morton
Extra Credit Reading:
- Li, J., Ross, D. (1993). Evidence for two kinds of hydrogen bond in ice. Nature, 365, 327–329. https://doi.org/10.1038/365327a0
- Garten, V. A., & Head, R. B. (1964). Hydrogen-bonding Patterns and Ice Nucleation. Nature, 204(4958), 573. https://doi.org/10.1038/204573a0
- Ishiyama, T., & Kitanaka, K. (2020). Asymmetric Hydrogen-Bonding Structure at a Water/Ice Interface. The Journal of Physical Chemistry C, 124(42), 23287–23294. https://doi.org/10.1021/acs.jpcc.0c08173

