We live in an amazing world! When we dig into the details of nature, we find things that surprise us, make us curious, and often leave us bewildered. Even our best scientists only understand small pieces of the complexity of the world. I believe that there is a God who not only understands it all, but invented it all. And in my own scientific training, I have found that God thought of everything.
DNA likely wins the contest for Most Famous Biomolecule, and for good reason. This specific chain of molecules carries almost all the information that a living thing needs, and like reading someone’s journal, we can learn a lot about life by searching its DNA. It is also virtually indestructible.
Chemically, it takes a lot to break DNA apart. Without the action of particular proteins designed to break the DNA chain at specific places at specific times, it basically does not happen. It’s why intact DNA can be extracted from fossils thousands and hundreds of thousands of years old, long after that DNA was done serving its purpose for its organism. Yet, an almost identical type of biomolecule–RNA–has a lifetime shorter than your coffee break.*
DNA and RNA are both nucleic acids. All nucleic acids are essentially chains of smaller units. To link together, one unit shares an oxygen atom with the neighboring unit. There is only one major difference between DNA and RNA when it comes to their chain links. See if you can spot the difference in the figure below!
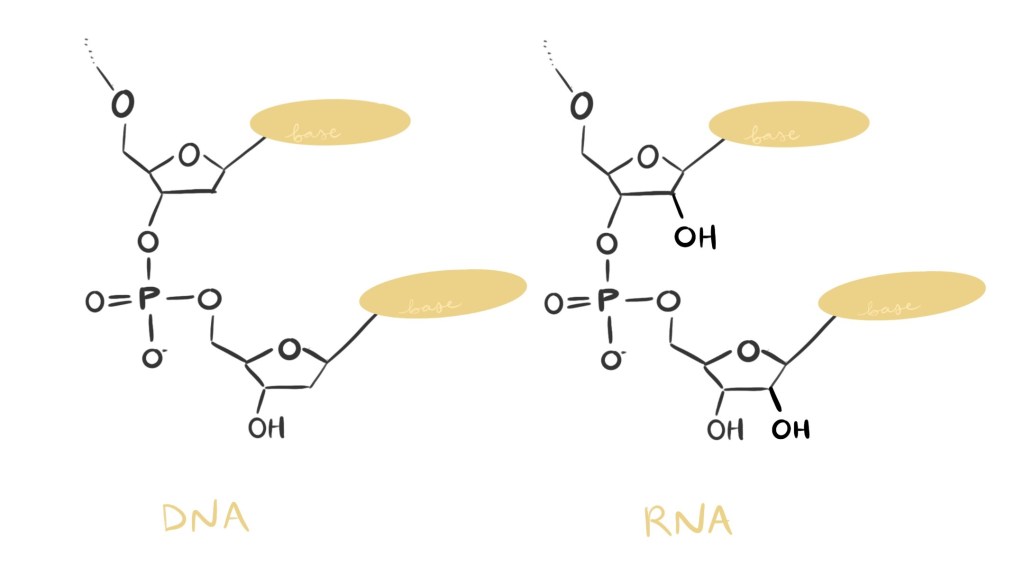
If you said, “I don’t know about the figure but R and D are different??” then you are still correct! The R in RNA stands for “ribose” and the D in DNA stands for “deoxyribose,” indicating that DNA has one fewer oxygen atom. That one missing oxygen atom makes all the difference in DNA’s lifetime.
In RNA molecules, when the oxygen atom in question (we’ll call it O’ since it’s special) is in the right “mood,” it reaches over and grab the phosphorous atom (P). Since P can only hold on to four atoms at a time, to accept O’, P lets go of another oxygen atom–the one it shares with its neighboring unit. P’s trade breaks the link between RNA units. And what gets O’ in the mood to break bonds? A lot of things common in watery environments like the ones in a cell.
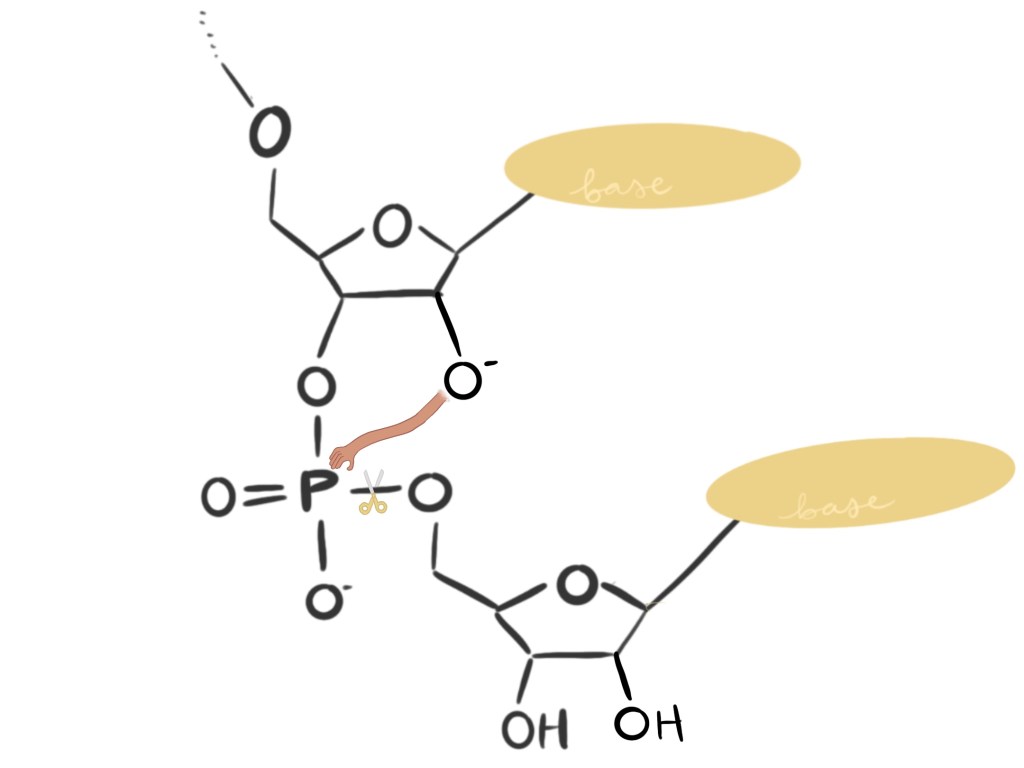
Since DNA is missing O’, the same common things that trigger O’ to sever RNA’s links have no effect on DNA’s links. While RNA can break apart spontaneously, DNA can’t. And isn’t that a relief?? Could you imagine if our DNA could just disintegrate in a matter of minutes without any specific signal? RNA’s short lifetime works well for its functions, which are largely time-bound and temporary, but if DNA didn’t last long, life would not last long either (if it could exist at all). RNA is made for a time, and DNA is made for a lifetime.
Dear God, thank you for withholding that one oxygen atom when you designed DNA! You’ve considered every chemical possibility in creating life, and made sure that all the biomolecules we need are there for as long as we need them. You know all things and you are infinitely wise in your creation. Amen.
*With some exceptions; see “Long-Lived RNAs” article
More About This Topic:
- DNA vs. RNA: https://www.technologynetworks.com/genomics/articles/what-are-the-key-differences-between-dna-and-rna-296719
- How long RNA lives: https://www.sciencedaily.com/releases/2017/07/170712201054.htm
- Long-Lived RNAs: https://www.genengnews.com/news/long-lived-rna-in-nerve-cells-can-last-a-lifetime/#:~:text=Lead%20authors%2C%20Martin%20Hetzer%2C%20PhD,two%2Dyear%20lifespan%20of%20the
Extra Credit Reading:
- Oivanen M, Kuusela S, Lönnberg H. Kinetics and Mechanisms for the Cleavage and Isomerization of the Phosphodiester Bonds of RNA by Brønsted Acids and Bases. Chem Rev. 1998 May 7;98(3):961-990. doi: 10.1021/cr960425x.
- Wayment-Steele HK, Kim DS, Choe CA, Nicol JJ, Wellington-Oguri R, Watkins AM, Sperberg RAP, Huang PS, Participants E, Das R. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Research, 2021 Oct. 11;49(18):10604–10617. doi: 10.1093/nar/gkab764.

